MOLECULAR REPAIR MECHANISMS USING ELECTROLYSIS (USGET) IN PATELLAR TENDONITIS
© 2013 SECOT. Publicado por Elsevier España, S.L.
Introduction
Patellar tendonitis affects a significant number of athletes whose common characteristic is performing jumps or ballistic movements.1 At present, tendonitis is considered as a degenerative, rather than an inflammatory process. Despite the fact that several therapeutic options have been described, none has become established as the standard.2,3
The use of experimental models based on the induction of tendonitis through collagenase (a metalloproteinase capable of breaking down the peptide links of collagen) has been applied in the past.4 The experimental study of tendonitis has previously been based on the assessment of proteins such as cytochrome C, Smac/Diablo, vascular endothelial growth factor (VEGF), its receptor 2 (VEGFR-2) and peroxisome proliferator-activated receptor gamma (PPAR-gamma) nuclear transcription factor. Cytochrome C is a monomeric protein capable of activating the caspases which trigger the last phases of apoptosis in tendinopathies.5 Smac/Diablo is a mitochondrial protein whose release into the cellular cytosol induces apoptosis, presumably following the same routes as cytochrome C.6 VEGF is a signal protein involved in angiogenesis and vasculogenesis which has shown a capacity to stimulate division and migration of endothelial cells in vitro.7 VEGFR-2 is a tyrosine-kinase receptor which acts as the most important mediator of the angiogenic response of VEGF.8 Lastly, PPAR-gamma, which belongs to the family of nuclear transcription factors (superfamily of steroid receptors), has been shown to cause a reduction of the inflammatory response.9
The Ultrasound-guided Electrolysis (USGET) causes non-thermal, electrolytic ablation which induces a controlled inflammatory response, enabling the activation of cellular mechanisms involved in phagocytosis and regeneration of damaged soft tissue.10
Since recent works have shown good clinical results using the technique under study,11 the objective of the present analysis was to employ immunodetection and electrophoresis techniques to investigate the molecular mechanisms of tissue response involved in the treatment with the USGET, following induction of tendonitis in Sprague Dawley rats through the use of collagenase.
Materials and method
The study was conducted using 24 female Sprague Dawley rats, aged 7 months and weighing approximately 300 g. The study fulfilled the ethical requirements of and was approved by the Bioethics Committee of the Medical School (A-1301314899794). The study followed the regulations contained in Royal Decree 1201/2005, from October 10th, on the protection of animals used for experimentation (BOE n.◦ 252. p. 34367—34391).
The animals were distributed into 4 groups: 6 control rats which did not undergo any intervention (control group), 6 rats injected with collagenase which did not undergo treatment with the USGET technique (collagenase group), 6 rats injected with collagenase which were treated with the USGET technique at 3 mA intensity (USGET-3 mA group) and 6 rats injected with collagenase and treated with the USGET at 6 mA intensity (USGET-6 mA group).
The USGET technique consisted in the ultrasound-guided application by a 0.32 mm needle of a continuous current through a specially designed.
Experimental model
We injected 50 µg of type I collagenase (Sigma-Aldrich Laboratories, St. Louis, MO, US) into the proximal region of the patellar tendon of the rats, causing tendonitis verified by ultrasound, following the protocol defined by the European Society of Musculoskeletal Radiology for the study of tendinopathies.12
In order to apply the USGET, we carried out 3 ultrasound-guided punctures of 4 s duration each in the proximal region of the patellar tendon of the rats, at an intensity of 3 or 6 mA, depending on the study group. The rats were sacrificed after 7 days and a sample of the tendon was surgically extracted following the standard procedure. We followed the Lowry13 method to determine protein concentrations in the tissue samples within ranges of 0.01-1 mg/ml, and analyzed the samples through immunodetection and spectrophotometry (> = 660 nm). We analyzed cytochrome C, Smac/Diablo, VEGF and VEGFR-2 proteins and also studied the PPAR-gamma) nuclear transcription factor. The results were validated by means of western blot studies versus tubulin and expressed as relative densitometry units.
Statistical analysis
The results were expressed as mean standard deviation. The statistical analysis was conducted through the Student t test. We carried out an ANOVA test to assess the relationships between the variables, as well as post hoc and Dunnett tests to compare the different groups with the control group and the Scheffé test to compare all the groups with each other. The level of statistical significance was set at 5% (P < .05). The statistical analysis was conducted using the software package SPSS® version 17 (SPSS Inc., Chicago, IL, US).
Results
The study of cytochrome C revealed elevated lev- els of this protein in all the groups being compared with the control group (P < .001). We observed statistically significant differences when comparing the USGET-3 mA group and the USGET-6 mA group (P < .013), and also when comparing the USGET-6 mA group and the collagenase group (P = .002).
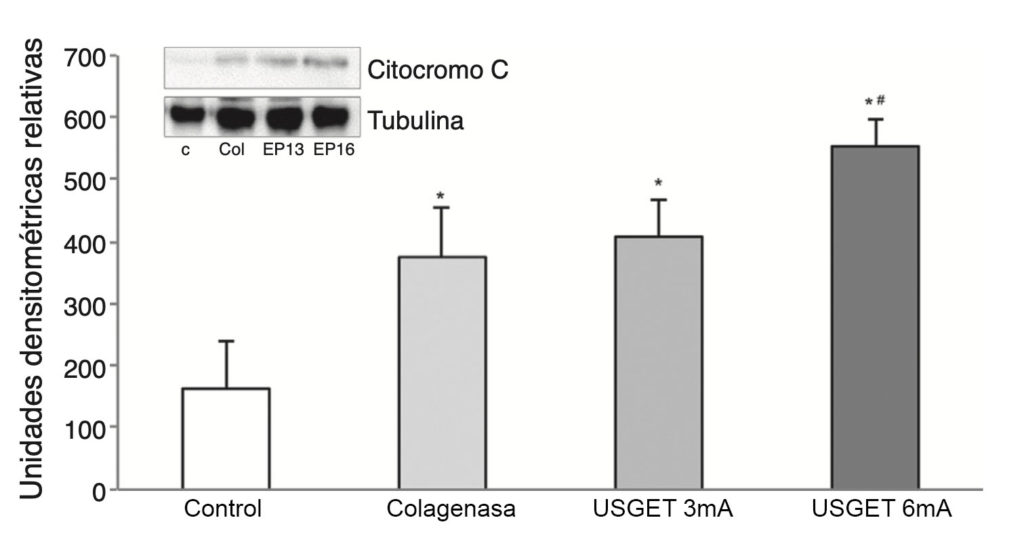
We also observed an overexpression of Smac/Diablo protein (P < .001), and detected statistically significant differences when comparing the 2 treatment groups (USGET- 3 mA and USGET-6 mA) with the collagenase group (P < .001).

The analysis of VEGF revealed a significant increase (P < .001) in all the studied groups. A significant increase (P < .001) of VEGFR-2 was also detected.

Lastly, there was a significant increase in PPAR-gamma levels compared to the control group (P < .001), with statistically significant differences being observed when comparing the USGET-3 mA (P = .009) and USGET-6 mA (P < .001) groups with the collagenase group.

Discussion
The main finding of this work was that the USGET produced an overexpression of cytochrome C, Smac/Diablo, VEGF and VEGFR-2 proteins, as well as the nuclear transcription factor PPAR-gamma).
Although several techniques have been described for the treatment of tendonitis, none is currently considered as the standard. Such techniques include excentric exercise, surgery (open or arthroscopic), shock waves, sclerosis of neovascularizations, non-steroidal anti-inflammatory drugs (NSAIDs) and the application of platelet rich plasma or aprotinin, among others.2,3
The USGET technique consists in a non-thermal electrical current which induces a regenerative response in the damaged tissue.10 The ionic instability leads to formation of sodium hydroxide molecules, thus modifying the pH and increasing oxygen under the active electrode or cathode needle. This in turn enables phagocytosis and biological activation of tendon repair, which was altered by the chronic degenerative process.10,11
Previous works with electrolytic therapy, such as that by Gravante et al.14 revealed the effects of these techniques on the inflammatory response. A metaanalysis conducted by Gardner et al.15 showed that electrical stimulation in chronic wounds and decubitus ulcers led to a faster healing, whilst Zhao et al.16 observed how electrical fields applied to endothelial cell cultures stimulated the production of VEGF, as well as cellular migration and elongation. These results are in accordance with those observed in the present work. Subsequently, Yang et al.17 reported that the application of direct currents in damaged soft tissues was essential to epithelial cell migration and management in the scarring response.
The theory of tendon lesions secondary to overuse seems to be the most widely accepted.1 Coinciding with authors such as Alfredson et al.18 and Tan and Chan,19 we consider tendonitis as a degenerative process, more than an inflam- matory process. In agreement with Fu et al.20 an increase in VEGF, Smac/Diablo, cytochrome C and VEGFR-2 proteins and anti-inflammatory protein PPAR-‘) is related to the inflammatory response and tissue repair. Since tendonitis is a degenerative process, treatment with the USGET could be justified.10,11,21-23
The present study revealed a greater overexpression capacity of cytochrome C, an apoptosis marker related to tendonitis,5 following application of the USGET. The Smac/Diablo protein was exported to the cytosol from the mitochondria, causing apoptosis through the activation of caspases6 and DNA damage resulting from binding to the CD95 receptor.24 The data obtained shows how the groups receiving treatment with the USGET technique presented an increase in the expression of this protein. As previously described by Huang et al.25 the increase of apoptosis via Smac/Diablo and induction of VEGF through VEGFR-2 is probably due to the increase of B cell inhibition in the development of the bone marrow and differentiation of T cells from the thymus.
An increase of anti-inflammatory proteins, like PPAR-gamma),9 has been observed after treatment with the USGET. These proteins play a key role in the inhibition of expression of proinflammatory molecules secreted by macrophages, such as TNF-a., IL-6 and IL-1 ,26 thus producing in the treated tissue a highly beneficial molecular response during tendonitis. This, in turn, results in an increase of the expression of VEGF and VEGFR-2, mediators responsible for angiogenesis anti-inflammatory response.7,27
The literature identifies the VEGFR-1 and VEGFR-2 receptors as the best expressed in the human Achilles tendon.8 Our results show an increase of VEGFR-2 following treatment with the USGET technique, showing a modification of the cellular apoptosis pathway and increase of angiogenesis.
A limitation of this study was the use of experimental models in animals, as the results obtained may not be fully extrapolated to humans.28 However, the results of this study are promising and highlight the need to conduct additional studies which include molecular microdialysis and histological studies of the treated tissue.18,29 It is worth pointing out the discreet number of experimental animals, although the results showed adequate statistical power. Another limitation could be the study of 6 molecular alterations in such a complex and poorly-known pathology.
Conclusions
In tendon lesions induced through type I collagenase in rats, the USGET technique produces an increase of anti- inflammatory and angiogenic molecular mechanisms.
References
- Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-7.
- Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res 2008;466:1539-1554.
- Larsson ME, Käll I, Nilsson-Helander K. Treatment of patellar tendinopathy – a systematic review of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc. 2012;20:1632-46.
- Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res. 2002;20:910-9.
- Yuan J, Murrell GA, Trickett A, Wang MX. Involvement of cytochrome c reléase and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta. 2003;1641:35-41.
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43-53.
- Sahin H, Tholema N, Petersen W, Raschke MJ, Stange R. Impaired biomechanical properties correlate with neoangiogenesis as well as VEGF and MMP-3 expression during rat patellar tendon healing. J Orthop Res. 2012;30:1952-7.
- Petersen W, Pufe T, Zantop T, Tillmann B, Tsokos M, Mentlein R. Expression of VEGFR-1 and VEGFR-2 in degenerative Achilles tendons. Clin Orthop Relat Res. 2004;420:286-91.
- de Mos M, Koevoet WJ, Jahr H, Verstegen MM, Heijboer MP, Kops N, et al. Intrinsic differentiation potential of adolescent human tendon tissue: an in-vitro cell differentiation study. BMC Musculoskelet Disord. 2007;8:16.
- Sánchez-Ibáñez JM. Evolución clínica en el tratamiento de la entesopatía rotuliana crónica mediante electro- estimulación percutánea ecodirigida: estudio de una serie de casos en población deportiva [tesis doctoral], León, Universidad de León, 2013.
- Sánchez- Sánchez JL. Estudio comparativo de un tratamiento fisioterápico convencional con uno que incluya la técnica Electrolisis Percutánea Intratisular® en pacientes con tendinopatía crónica del tendón rotuliano [tesis doctoral], Salamanca, Universidad de Salamanca, 2011.
- Beggs I, Bianchi S, Bueno A, Cohen M, Court-Payen M, Grainger A, et al. En: ESSR Ultrasound Group Protocols. Musculoskeletal Ultrasound Technical Guidelines: Knee. p. 3.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75.
- Gravante G, Ong SL, Metcalfe MS, Sorge R, Overton J, Lloyd DM, et al. Cytokine response of electrolytic ablation in an ex vivo perfused liver model. ANZ J Surg. 2010;80:537-41.
- Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen. 1999;7:495-503.
- Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397-405.
- Yang K, Zhao Z, Gross RW, Han X. Systematic analysis of choline-containing phospholipids using multi-dimensional mass spectrometry-based shotgun lipidomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2924-36.
- Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique–no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71:475-9.
- Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-15.
- Fu SC, Rolf C, Cheuk YC, Lui PP, Chan KM. Deciphering the pathogenesis of tendinopathy: a three-stages process. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:30.
- Lian Ø, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605-11.
- Scott A, Lian Ø, Bahr R, Hart DA, Duronio V, Khan KM. Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med. 2008;42:753-7.
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457-60.
- Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46:497-510.
- Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624-31.
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82-6.
- Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res. 2006;24:393-400.
- Lui PP, Maffulli N, Rolf C, Smith RK. What are the validated animal models for tendinopathy? Scand J Med Sci Sports. 2011;21:3-17.
- Maffulli N, Del Buono A, Spiezia F, Longo UG, Denaro V. Light microscopic histology of quadriceps tendon ruptures. Int Orthop. 2012;36:2367-71.



 Abat F, et al.
Abat F, et al.