CLINICAL UTILITY OF DIAGNOSTIC ULTRASOUND IN ATHLETES
WITH TENDINOPATHY (ICL 22)
 Ferran Abat, Nicola Maffulli, H. Alfredson, Lopez-Vidriero, C. Myers, S. Gomes, and O. Chan
Ferran Abat, Nicola Maffulli, H. Alfredson, Lopez-Vidriero, C. Myers, S. Gomes, and O. Chan
R. Becker et al. (eds.), ESSKA Instructional Course Lecture Book: Barcelona 2016, DOI 10.1007/978-3-662-49114-0_19
Introduction
Chronic painful tendinopathy is common in elite and recreational athletes and in sedentary sub- jects; all may have to stop or decrease their level of physical activity [1, 2]. Midportion Achilles tendinopathy and for the younger and heavy loading population also patellar tendinopathy are problematic injuries. However, recent research on innervation patterns histopathology and pain mechanisms in Achilles and patellar tendons has led to an increased knowledge about the chronic painful tendon [3–6].
Classically, the term ‘tendinitis’ was used considering that the fundamental lesion was an inflammation of the tendon. However, by the time these lesions become clinically evident, at histology there is an absence of inflammatory cells. Instead, the injured tissue presents frag- mentation, an alteration of the collagen and vascular hyperplasia [7–9], and a pathological picture compatible with a failed healing response.
Better, though still incomplete, understanding of the pathophysiology of tendinopathy has induced changes in the therapeutic approach used in the management of tendinopathy. Most authorities have abandoned the goal of eliminating inflammation of the tendon and tried to impact on the biology of the tendon to stimulate its regeneration [10]. Chronic pathologies are characterised histologically by irregular tendon structure with a failed healing response, with the presence of numerous fibroblasts and pathological neovascularisation [11].
Ultrasound and colour Doppler findings [12], showing localised high blood flow inside and outside regions with structural tendon abnormalities, have shown to be important to diagnose tendinopathy [13–15]. Immunohistochemical analyses of biopsies have shown sensory and sympathetic nerves in close relation to the outside of the tendon. These findings have led to the development of new treatment methods. Alfredson et al. [15] suggested that these new vessels and nerves were involved in the mechanisms of tendinopathy pain, but the answer as to the origin of the pain is an issue that is still under debate.

Fig. Patellar tendinopathy studied with high-definition colour Doppler ultrasound. Comparison between right and left patellar tendons. Longitudinal view reveals intensive thickening (11 mm vs. 3.9 mm) com- bined with hypoechoic zones.
Can US Replace MRI in the Diagnosis and Management of Tendinopathies?
Currently, ultrasound is the imaging modality of choice for the assessment of tendons as it has superior spatial resolution to MRI and ultrasound and colour doppler examination can be used to diagnose partial ruptures. However, clinical improvement is not correlated with changes in imaging status or the amount of neovascularity [16].
Also, ultrasound is dependent on the skills of the operator and, as MRI, produces a bidimensional image of a tridimensional structure. This may introduce limitations in assessing the structural integrity of the tendon.

Fig. UTC imagines showing 55 % type 1 echoes (green) when normal is approximately 70 %. The tendon has areas of matrix degeneration. This area is demonstrated at 1–1.5 cm from the calcaneum (black arrows) on the ventral aspect of the tendon and is demonstrated by the focal area of type 3 echoes (red)
A new novel imaging modality ultrasound tissue characterisation (UTC™) provides a more detailed imaging profile of the tendon. UTC imaging produces a multiplanar and 3D coronal view to assess in detail the structural integrity of the tendon [17]. These ultrasonographic images provide objective information on the integrity of the tendon matrix from the distal insertion to musculotendinous junction. The scans are analysed to assess for focal areas of echo change and to establish the overall health of the tendon.
UTC may play an important role in monitoring athletes’ tendon health during each phase of the rehabilitation process and for managing in-season tendon pain. Managing tendinopathy during the competitive season is particularly challenging as training and competition loads are high and often there is not sufficient time for a full recovery. Excessive loading provokes tendon pain: the greater the load, the greater the pain experienced [18]. UTC data combined with clinical markers assess the tendon tolerance to load, such as 24-h pain response, morning stiffness, pain on single- leg heel raise, and single-leg hops. This information is used to adjust and modify tendon load to ensure that the tensile-loading capabilities of the tendon are not exceeded and the tendon remains pain-free. This enables athletes, their clinicians and coaches to make informed and effective deci- sions about the capacity for training and performance. Research has also demonstrated that UTC is valid, reliable and sensitive at detecting a tissue response to load [19, 20].
Neovascularity in Tendinopathy
In the 1990s, Newman et al. [21] described blood flow in symptomatic tendons at power Doppler ultrasonography. Subsequently, Ohberg and Alfredson [22] defined this blood flow as ‘neovascularisation’. From an etiological perspective, the neovessels were thought to be secondary to the essential abnormality of tendinopathy, the failed healing lesion [23]. Using colour Doppler ultrasound, Ohberg and Alfredson showed, in a case-control study, increased blood flow and neovascularity in all painful tendons and absence of these features in the asymptomatic control tendons [15]. Healthy tendons are relatively avascular [24].
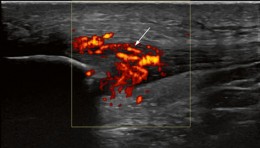
Fig. US image of patellar tendinopathy with neurovascular ingrowth.
Symptomatic tendinopathic Achilles tendons with neovascularisation show evidence of a statistically significant association between the site of maximum tenderness on palpation and the site of maximum presence of neovessels [25]. Also, neovessels were detected in 29 % of asymptomatic athletes [26] and in 100 % of subjects after strenuous exercise [27].
Recent research, it appears that detecting neovessels may have no additional value for the diagnosis, no firmly confirmed prognostic value and no proven relation with symptoms [28]. Also, all these issues can be compounded by the lack of standardisation of machine settings regarding the use of power or colour Doppler [29].
Injection Therapies in Tendinopathy
Injection therapies include a range of options such as corticosteroids, high-volume saline, prolotherapy, autologous blood, platelet-rich plasma, aprotinin, botulinum toxin, sodium hyaluronate, polysulphated glycosaminoglycan and polidocanol [30].
Injection therapies can be guided by real-time ultrasound imaging or performed ‘blind’, they can be administered in isolation or in combination with any of the above interventions, they can be administered in a single dose or consist of a course and they can be injected locally into the tendon or targeted at specific sites (such as areas of vascular ingrowth). There is no consensus on many of these factors and the exact intervention is at the discretion of the responsible clinician [31]. Some injection therapies are used to deliver a drug directly to the damaged tendon while others like polidocanol are to be injected outside the tendon in specific regions. In general, these sub- stances are thought to act either pharmacologically (e.g. corticosteroids or polidocanol) or mechanically (e.g. high-volume saline to disrupt neovascular growth) [32].
Ultrasound-Guided Mini Surgery for Tendinopathy Treatment
Originally, ultrasound Doppler-guided injections of the sclerosing substance polidocanol [33, 34] targeting the regions with high blood flow outside the tendon were used. The clinical results were good, but often multiple injections during a 3–6 months period of time were needed. Also, the procedure is technically demanding with a relatively long learning curve. However, using this method lot of knowledge about the location for pain was achieved. This knowledge was used when moving into mini-invasive surgical methods [35, 36]. For the chronic painful tendinopathy of the main body of the Achilles tendon, an ultrasound Doppler-guided scraping technique, targeting the regions with high blood flow and nerves on the ventral side of the tendon, was described. The procedure is indicated when 3 months of heavy loaded painful eccentric train- ing have failed and has been shown to be very successful in elite athletes as well as recreational athletes and sedentary patients. Very few complications are reported, but proper wound care needs to be emphasised. Early (4–6 weeks) return to heavy tendon loading sport activities was obtained. In follow-up studies, remodelling of the tendon structure over time was seen. We are now starting to use a percutaneous surgical technique allowing for an even earlier return to activity. Recently, plantaris tendon involvement in midportion Achilles tendinopathy has been high- lighted [37–39].
In a subgroup of patients, often complaining of localised medial tendon pain, and having a poor result of eccentric training, a nearby, some- times invaginated, plantaris tendon can be of importance for the pain. The plantaris tendon can be tendinopathic, and the paratendinous tissues between the Achilles and plantaris tendons were richly innervated. Often, also the plantaris tendon itself was richly innervated. In patients with mid- portion Achilles tendinopathy and a suspected plantaris tendon involvement, surgical treatment is instituted early. Ultrasound Doppler-guided removal of the plantaris tendon, together with the scraping procedure for the Achilles, is used. The clinical results have been shown to be very good, with an early return to heavy tendon loading activities. Follow-up studies have shown a quick remodelling of the medially located structural abnormalities in the midportion of the Achilles tendon, indicating a possible compressive or shearing disturbance from the plantaris tendon.
For patients with patellar tendinopathy jumper’s knee in the proximal patellar tendon, ultra- sound Doppler-guided arthroscopic shaving technique, targeting the regions with high blood flow and nerves on the dorsal side of the proximal tendon, has been invented [40, 41]. The method is used when 3 months of heavy loaded painful eccentric training has failed and has been shown to be very successful in elite and recreational athletes. Very few complications were reported, with an early (6–8 weeks) return to heavy tendon loading sport activities. In follow- up studies, remodelling of the tendon structure over time was seen.
Autologous US-Guided Treatments in Tendinopathy: How Should It Be Done?
Platelet-rich plasma (PRP) is a general term for new technologies that are focused on enhancing the healing response after injury of different tis- sue types [42, 43].
Tendons have low basal metabolic rates and are predisposed to slow healing after injury [44]. Basic science studies have shown that co-cultures of tenocytes and a preparation rich in growth factors increase the proliferation and secretion of VEGF and hepatocyte growth factor [45].
PRP has also proven to be effective in treating chronic tendinopathies. Mishra and colleagues showed a significant reduction, at 8 weeks, in tennis elbow symptoms in a group treated with PRP compared with a control group. A group from the Netherlands led by Gosen [46] has replicated this protocol and compared the PRP group with a group treated with cortisone injection for tennis elbow. They observed that the PRP group enjoyed better and faster functional recovery and pain relief after 6 months.
Marcacci and colleagues [47] have studied the effects of PRP in jumper’s knee (chronic refractory patellar tendinopathy) after previous classical treatments have failed. They observed significantly better results in terms of Tegner, EuroQol and visual analogue scale scores and pain level compared with baseline and with controls treated with physiotherapy.
Through the actual research, it is hard to draw any clear conclusion for the effectiveness of PRP treatment in terms of tendinopathy [48]. In case of PRP use, the treatment protocol consists of applying PRP under ultrasound control and filling the gap (if needed) under strictly ultra- sound guidance.
Take-Home Message
- Accurate clinical diagnosis is the key: be specific and consider all differential diagnoses.
- Carry out a detailed examination with a thorough history and ultrasound examination.
- Before commencing a loading programme, consider the irritability of the tendon. Monitor overall load on the tendon.
- Eccentric loading may be effective but con- sider other types of treatments when eccentric fails.
- Standardise the ultrasound examination and study the presence of high blood flow.
- Think about the use of ultrasound-guided minimally invasive techniques explained or surgery when appropriate rehabilitation has not given good results.
References
Andia I, Maffulli N. Muscle and tendon injuries: the role of biological interventions to promote and assist healing and recovery. Arthroscopy. 2015;31(5):999–1015.
Abat F, Sanchez-Ibañez JM. Patellar tendinopathy: a critical review of current therapeutic options. OA Sports Med. 2014;2(1):2.
Bjur D, Alfredson H, Forsgren S. The innervation pat- tern of the human Achilles tendon: studies of the nor- mal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005; 320(1):201–6.
Danielson P, Alfredson H, Forsgren S. Distribution of general (PGP 9.5) and sensory (substance P/CGRP) innervations in the human patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):125–32.
Andersson G, Danielson P, Alfredson H, Forsgren S. Nerve-related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1272–9.
Danielson P, Andersson G, Alfredson H, Forsgren S. Marked sympathetic component in the perivascular innervation of the dorsal paratendinous tissue of the patellar tendon in arthroscopically treated tendinosis patients. Knee Surg Sports Traumatol Arthrosc. 2008;16(6):621–6.
Khan KM, Cook JL, Kannus P, Maffulli N, Bonar SF. Time to abandon the “tendinitis” myth. BMJ. 2002; 324(7338):626–7.
Khan KM, Cook JL, Taunton JE, Bonar F. Overuse tendinosis, not tendinitis part 1: a new paradigm for a difficult clinical problem. Phys Sportsmed. 2000; 28(5):38–48.
Cook JL, Khan KM, Maffulli N, Purdam C. Overuse tendinosis, not tendinitis part 2: applying the new approach to patellar tendinopathy. Phys Sportsmed. 2000;28(6):31–46.
Cook JL, Purdam CR. Is tendon pathology a contin- uum? A pathology model to explain the clinical pre- sentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–16.
Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27(6):393–408.
Malliaras P, Purdam C, Maffulli N, Cook J. Temporal sequence of greyscale ultrasound changes and their relationship with neovascularity and pain in the patel- lar tendon. Br J Sports Med. 2010;44(13):944–7.
Tol JL, Spiezia F, Maffulli N. Neovascularization in Achilles tendinopathy: have we been chasing a red herring? Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1891–4.
Malliaras P, Richards PJ, Garau G, Maffulli N. Achilles tendon Doppler flow may be associated with mechani- cal loading among active athletes. Am J Sports Med. 2008;36(11):2210–5.
Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diag- nostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):334–8.
De Jonge S, Warnaars JL, De Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Tol JL. Relationship between neovascularization and clin- ical severity in Achilles tendinopathy in 556 paired measurements. Scand J Med Sci Sports. 2014;24(5): 773–8.
vanSchie HT, de Vos RJ, de Jonge S, Bakker EM, Heijboer MP, Verhaar JA, Tol JL, Weinans H. Ultrasonographic tissue characterisation of human Achilles tendons: quantification of tendon structure through a novel non-invasive approach. Br J Sports Med. 2010;44(16):1153–9.
Kountouris A, Cook J. Rehabilitation of Achilles and patellar tendinopathies. Best Pract Res Clin Rheumatol. 2007;21(2):295–316.
Rosengarten SD, Cook JL, Bryant AL, Cordy JT, Daffy J, Docking SI. Australian football players’ Achilles tendons respond to game loads within 2 days: an ultrasound tissue characterisation (UTC) study. Br J Sports Med. 2015;49(3):183–7.
Docking SI, Daffy J, van Schie HT, Cook JL. Tendon structure changes after maximal exercise in the Thoroughbred horse: use of ultrasound tissue charac- terisation to detect in vivo tendon response. Vet J. 2012;194(3):338–42.
Newman JS, Adler RS, Bude RO, Rubin JM. Detection of soft-tissue hyperemia: value of power Doppler sonography. AJR Am J Roentgenol. 1994;163(2): 385–9.
Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9(4): 233–8.
Cook JL, Khan KM, Purdam C. Achilles tendinopa- thy. Man Ther. 2002;7(3):121–30.
Sharma P, Maffulli N. Basic biology of tendon injury and healing. Surgeon. 2005;3(5):309–16.
Divani K, Chan O, Padhiar N, Twycross-Lewis R, Maffulli N, Crisp T, Morrissey D. Site of maximum neovascularisation correlates with the site of pain in recalcitrant mid-tendon Achilles tendinopathy. Man Ther. 2010;15(5):463–8.
Sengkerij PM, de Vos RJ, Weir A, van Weelde BJ, Tol JL. Interobserver reliability of neovascularization score using power Doppler ultrasonography in mid- portion achilles tendinopathy. Am J Sports Med. 2009;37(8):1627–31.
Boesen MI, Boesen A, Koenig MJ, Bliddal H, Torp- Pedersen S. Ultrasonographic investigation of the Achilles tendon in elite badminton players using color Doppler. Am J Sports Med. 2006;34(12):2013–21.
Malliaras P, Chan O, Simran G, MartinezdeAlbornoz P, Morrissey D, Maffulli N. Doppler ultrasound signal in Achilles tendinopathy reduces immediately after activity. Int J Sports Med. 2012;33(6):480–4.
Yang X, Pugh ND, Coleman DP, Nokes LD. Are Doppler studies a useful method of assessing neovas- cularization in human Achilles tendinopathy? A sys- tematic review and suggestions for optimizing machine settings. J Med Eng Technol. 2010;34(7–8): 365–72.
Chan O, O’Dowd D, Padhiar N, Morrissey D, King J, Jalan R, Maffulli N, Crisp T. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil. 2008;30(20–22):1697–708.
Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010; 376(9754):1751–67.
Kearney RS, Parsons N, Metcalfe D, Costa ML. Injection therapies for Achilles tendinopathy. Cochrane Database Syst Rev. 2015;(5):CD010960.
Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005; 13(4):338–44.
Lind B, Ohberg L, Alfredson H. Sclerosing polidoca- nol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1327–32.
Alfredson H, Ohberg L, Zeisig E, Lorentzon R. Treatment of midportion Achilles tendinosis: similar clinical results with US and CD-guided surgery out- side the tendon and sclerosing polidocanol injections. Knee Surg Sports Traumatol Arthrosc. 2007;15(12): 1504–9.
Alfredson H. Ultrasound and Doppler-guided mini- surgery to treat midportion Achilles tendinosis: results of a large material and a randomised study comparing two scraping techniques. Br J Sports Med. 2011; 45(5):407–10.
Alfredson H. Midportion Achilles tendinosis and the plantaris tendon. Br J Sports Med. 2011;45(13):1023–5.
Spang C, Harandi VM, Alfredson H, Forsgren S. Marked innervation but also signs of nerve degenera- tion in between the Achilles and plantaris tendons and presence of innervation within the plantaris tendon in midportion Achilles tendinopathy. J Musculoskelet Neuronal Interact. 2015;15(2):197–206.
Masci L, Spang C, van Schie HTM, Alfredson H. Achilles tendinopathy-does plantaris tendon removal and Achilles scraping improve tendon structure? A prospective study using ultrasound tissue characteri- sation. BMJ Open. 2015;1, e000005.
Willberg L, Sunding K, Forssblad M, Fahlström M, Alfredson H. Sclerosing polidocanol injections or arthroscopic shaving to treat patellar tendinopathy/ jumper’s knee? A randomised controlled study. Br J Sports Med. 2011;45(5):411–5.
Sunding K, Willberg L, Werner S, Alfredson H, Forssblad M, Fahlström M. Sclerosing injections and ultrasound-guided arthroscopic shaving for patellar tendinopathy: good clinical results and decreased ten- don thickness after surgery-a medium-term follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015; 23(8):2259–68.
Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the heal- ing environment. Arthroscopy. 2010;26(2):269–78.
Andia I, Sánchez M, Maffulli N. Platelet rich plasma therapies for sports muscle injuries: any evidence behind clinical practice? Exp Opin Biol Ther. 2011;11(4):509–18.
Sánchez M, Anitua E, Orive G, Mujika I, Andia Platelet-rich therapies in the treatment of orthopae- dic sport injuries. Sports Med. 2009;39(5):345–54.
Anitua E, Sanchez M, Orive G, Andia I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28:4551–60.
Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6): 1200–8.
Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34(6):909–15.
de Vos RJ, van Veldhoven PL, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010;95:63–77.